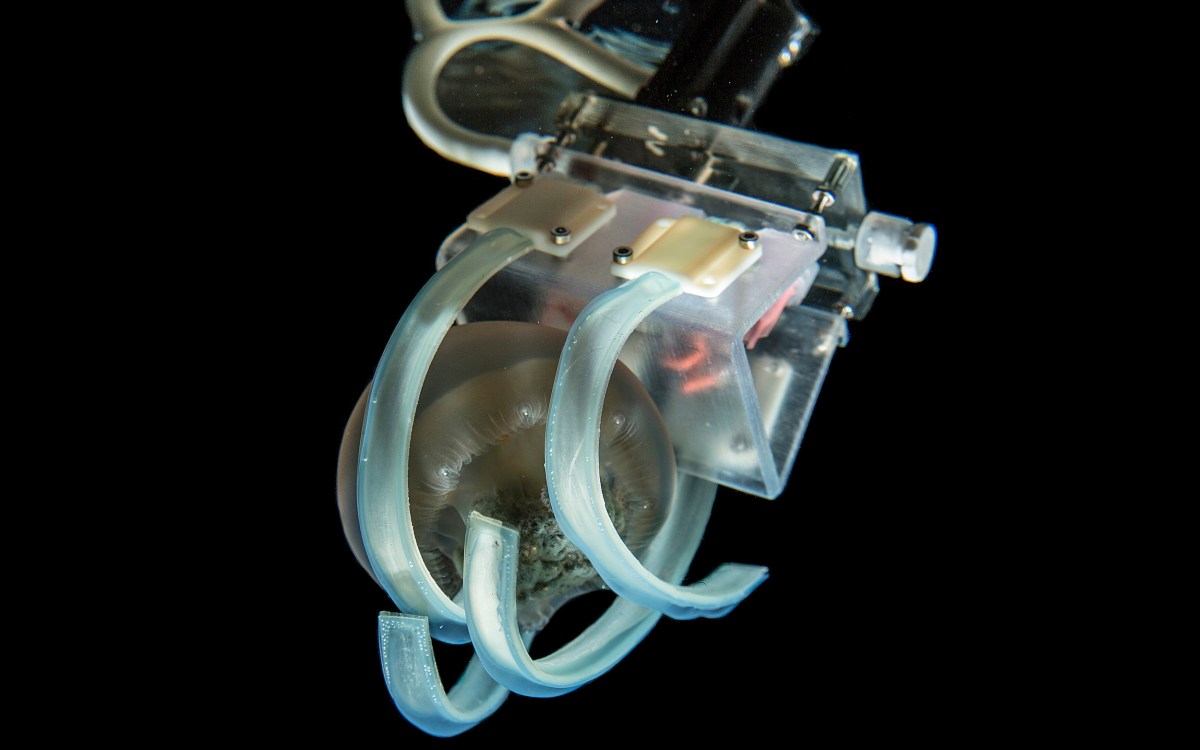
The upside-down jellyfish points its stinging cell-filled tentacles toward the water column to capture prey and deter predators.
Video by Lena van Giesen
How jellyfish and sea anemones know when (and when not) to sting
Scientists analyze stinging response on a molecular level
To sting or not to sting? For jellyfish, that is the question whenever their tentacles brush up against anything, including millions of human swimmers around the world.
Stingers are fired out at about the speed of a discharged bullet. And each single specialized cell responsible for a response can only be deployed once, as they rupture when used and have to be grown back after a jellyfish ejects its venom-coated barb into an unsuspecting prey or an unlucky swimmer. Given the limitations on its arsenal, it would seem some prudence is in order.
“To prevent unnecessary stinging [including of itself], there must be some kind of signal that allows the cell to shoot at the right time,” said Nicholas Bellono, assistant professor of molecular and cellular biology in the Faculty of Arts and Sciences. How that stinging trigger and safeguard system works on a molecular level in jellyfish and sea anemones has long been a mystery to scientists. At least, it was until a team of researchers from Bellono’s lab solved it.
They identified how the stinging cells, called nematocytes, which are found along the tentacles of sea anemones and jellyfish — both types of cnidaria — detect and filter diverse cues from the environment to control when (and when not) to sting.
The researchers found that nematocyte cells from the starlet sea anemone, a relation of the jellyfish, have an unusual calcium electrical current that is critical for initiating the stinging response, but that the ion channel controlling this current only opens under very specific conditions: a combination of mechanical stimuli from a tentacle making contact with a prey or predator, like a poke, and the presence of certain chemical cues, like those from prey or predators.
During all other times, these calcium channels are inactive and render the cell dormant until the right signal approaches.

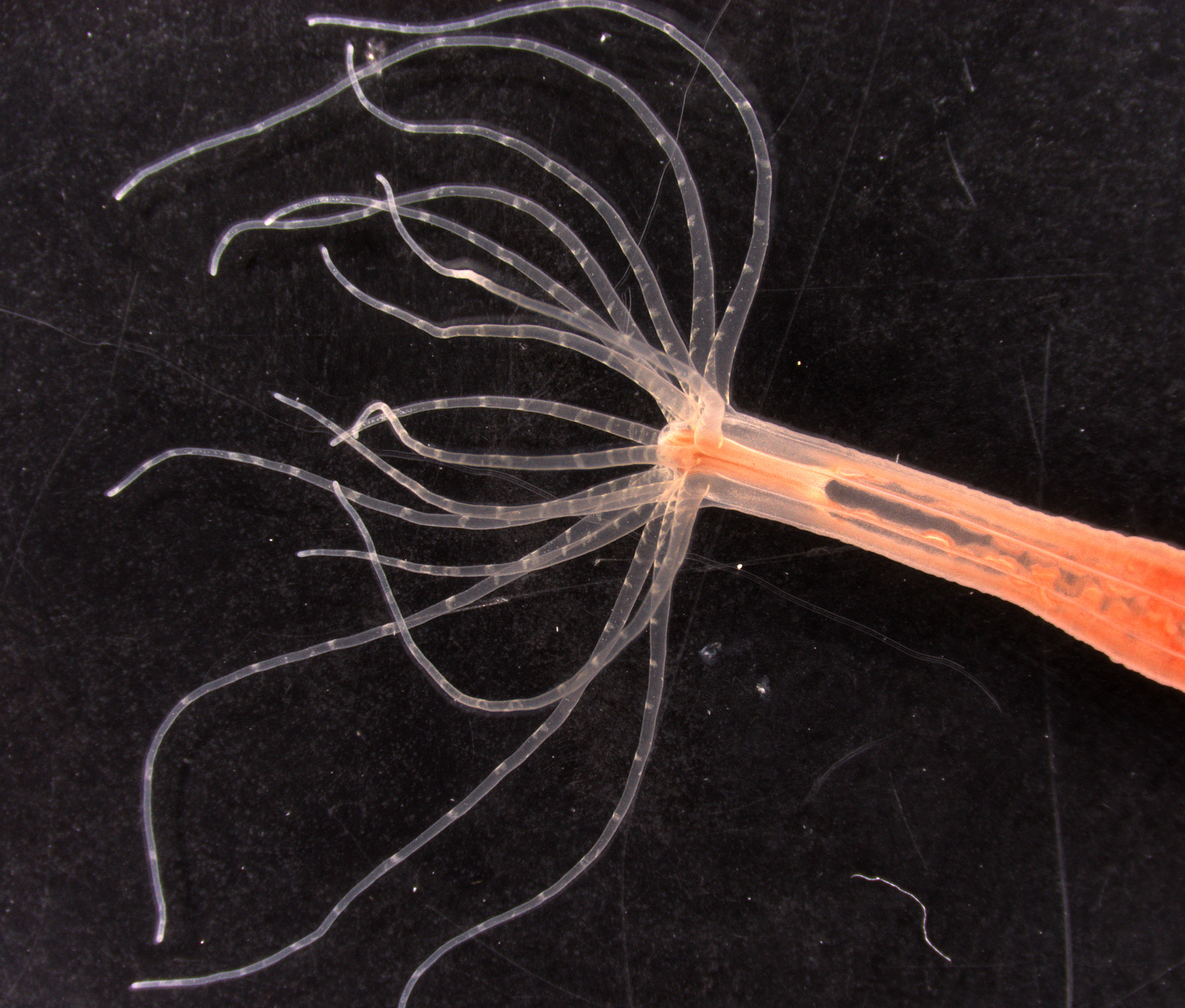
The starlet sea anemone uses specialized stinging cells to detect changes in its environment and fire a toxic barb to pierce and envenomate prey. The stinging cells in the tentacles use a unique ion channel to trigger stinging in response to prey.
Christophe Dupre and Keiko Weir
“We hypothesize that first, the sea anemone detects chemicals from its prey using chemosensory cells,” said Keiko Weir, a graduate research fellow who led the project. “These chemosensory cells then relay this information to nematocytes using acetylcholine [an organic chemical that acts as a neurotransmitter]. The acetylcholine relieves inactivation of these calcium channels. This functions to prime the nematocyte to say, ‘There’s food nearby.’ Then, once the nematocyte receives a mechanical cue, such as the tentacle contacting prey, that leads to the opening of the calcium channels, resulting in a huge calcium influx and the discharge of the nematocyte.”
Previous studies had already demonstrated that only the right combination of cues trigger nematocytes to fire, but the molecular process was unknown. The findings put it all together and highlight how nature has continuously developed elegant but simple systems for dealing with complex problems that call for ultrafast decision-making.
“The underlying principles of any biological system is you have cells that have to take cues from their surroundings — either from other cells or directly from the environment — and translate that information into an appropriate response,” Weir said.
What makes this system stand out in particular is that the final say of whether to sting comes down to the nematocyte.
“It’s a great example of when a single cell has to properly integrate the right signals in order to make a correct (and very extreme) decision,” Bellono said. “We’re often thinking about systems-level questions in which the brain makes complex computations using several components of a circuit, but this study helps demonstrate that each protein and each cell is critical to such processing because it comes down to one molecule having just the right properties to fit its cellular and organismal context.”
Along with Weir and Bellono, other co-authors included Christophe Dupre, a postdoctoral fellow from the Engert and Lichtman Lab; Lena van Giesen, a postdoctoral fellow in the Bellono lab; and Amy Lee, an assistant professor at Harvard Medical School. The study published in eLife in May.
The team used a variety of techniques, including physiology, behavior, and electron microscopy, that allowed them to meticulously follow the electrical and chemical processes leading to the stinging response.
As to why jellyfish sting an estimated 150 million people each year when humans are not its prey, the best answer is still likely a defense response. It could also lie in our chemical makeup, however.
“This comes back to which chemicals are sensed,” Bellono said. “Is the animal adapted to very broadly sense some generalized chemical that’s present in many animals such as us, even though we’re not prey? There are examples of sea anemones which use specific nematocytes for predation and others for defense. There are other animals which may use chemicals to avoid stinging, such as the clownfish. Maybe those nematocytes are tuned to specific chemical inputs.”
This research was supported by the New York Stem Cell Foundation, the Searle Scholars Program, the Sloan Foundation, the Klingenstein-Simons Fellowship, the National Institutes of Health, and the Swiss National Science Foundation.