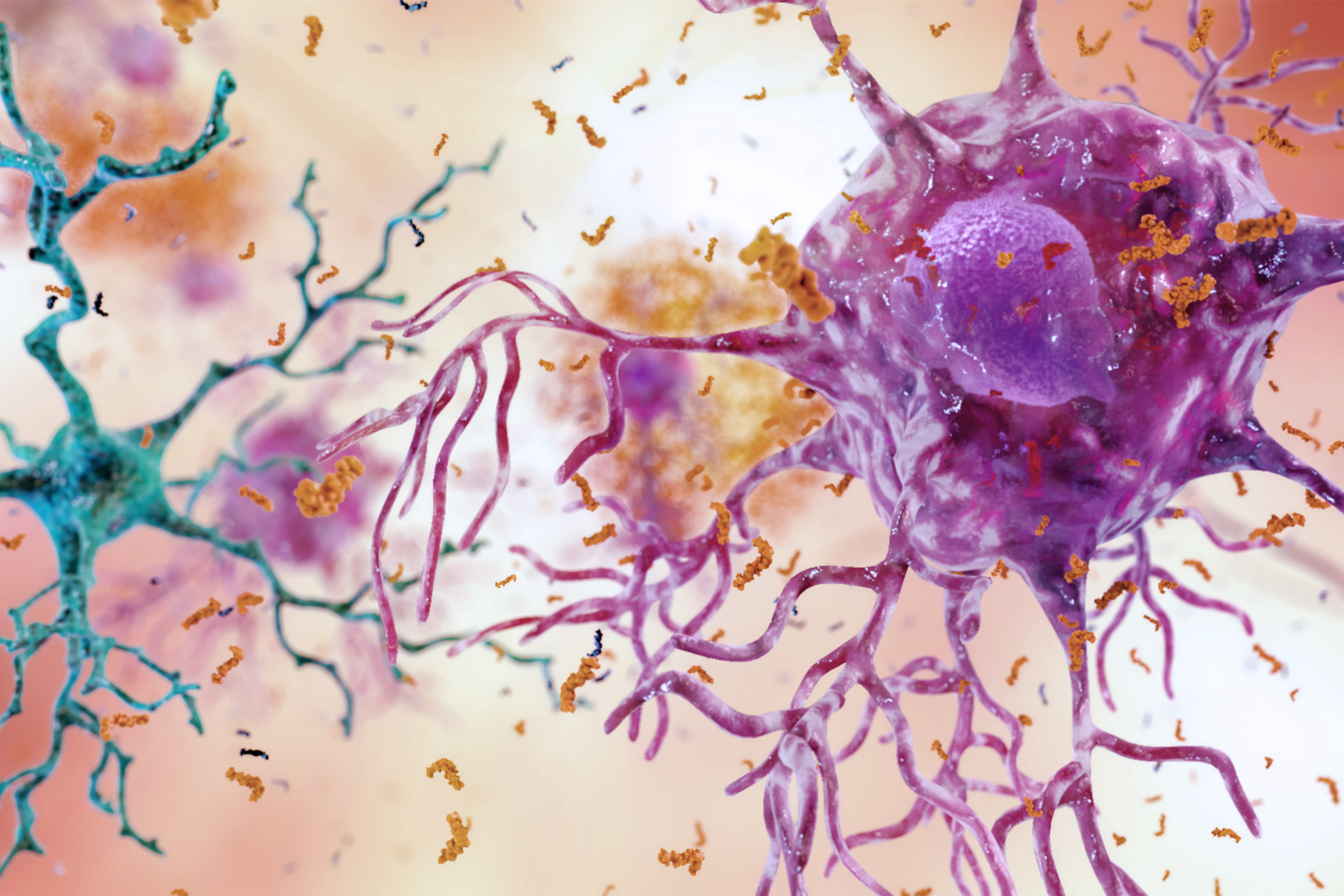
Cognition within the brain can be blocked by an inflammatory environment (pictured) causing further damage for those with Alzheimer’s disease. Based on a mouse model, exercise has shown new nerve cells to not only to survive, but thrive.
Image courtesy of the National Institute on Aging/NIH
Exercise can ‘clean up’ Alzheimer’s environment
Study shows exercise generates new neurons, improves cognition in mouse model
A study by a Massachusetts General Hospital (MGH) research team finds that neurogenesis — inducing the production of new neurons — in the brain structure in which memories are encoded can improve cognitive function in a mouse model of Alzheimer’s disease. Their investigation shows that cognition can be blocked by the hostile inflammatory environment in the brains of patients with Alzheimer’s disease and that physical exercise can “clean up” that environment, allowing new nerve cells to survive and thrive and improving cognition in the Alzheimer’s mice.
“In our study we showed that exercise is one of the best ways to turn on neurogenesis and then, by figuring out the molecular and genetic events involved, we determined how to mimic the beneficial effects of exercise through gene therapy and pharmacological agents,” said Rudolph Tanzi, director of the Genetics and Aging Research Unit, vice chair of the Department of Neurology, and co-director of the Henry and Allison McCance Center for Brain Health at MGH. Tanzi is the senior author of the paper published in Science.
Lead author Se Hoon Choi of the Genetics and Aging Research Unit said, “While we do not yet have the means for safely achieving the same effects in patients, we determined the precise protein and gene targets for developing ways to do so in the future.”
Adult neurogenesis — production of new neurons after the embryonic and, in some animals, neonatal periods — takes place in the hippocampus and another brain structure called the striatum. While adult hippocampal neurogenesis is essential to learning and memory, how the process impacts neurodegenerative conditions like Alzheimer’s has not been well understood. The MGH team set out to investigate how impairment of adult hippocampal neurogenesis (AHN) contributed to Alzheimer’s pathology and cognitive function in a mouse model, and whether increasing AHN could reduce symptoms.
“It is not enough just to turn on the birth of new nerve cells; you must simultaneously ‘clean up’ the neighborhood in which they are being born …. Exercise can achieve that …”
Rudolph Tanzi, senior author
Their experiments showed that AHN could be induced in the model either by exercise or by treatment with drugs and gene therapy that promoted the birth of neural progenitor cells. Behavioral testing on animals revealed limited cognitive benefits in animals in which neurogenesis had been induced pharmacologically and genetically. But animals in which AHN was induced by exercise showed improved cognitive performance and reduced levels of beta-amyloid, the main component of plaques in Alzheimer’s brains.
“Although exercise-induced AHN improved cognition in Alzheimer’s mice by turning on neurogenesis, trying to achieve that result by using gene therapy and drugs did not help,” Tanzi said. “That was because newly born neurons, induced by drugs and gene therapy, were not able to survive in brain regions already ravaged by Alzheimer’s pathology, particularly neuroinflammation. So we asked how neurogenesis induced by exercise differs.”
Choi explained, “We found the key difference was that exercise also turned on the production of brain-derived neurotrophic factor, or BDNF — known to be important for the growth and survival of neurons — which created a more hospitable brain environment for the new neurons to survive. By combining drugs and gene therapy that both induced neurogenesis and increased BDNF production, we were able to successfully mimic the effects of exercise on cognitive function.” Choi is an assistant professor of neurology at Harvard Medical School (HMS).
Tanzi added, “The lesson learned was that it is not enough just to turn on the birth of new nerve cells; you must simultaneously ‘clean up’ the neighborhood in which they are being born to make sure the new cells survive and thrive. Exercise can achieve that, but we found ways of mimicking those beneficial cognitive effects by the application of drugs and gene therapy that simultaneously turn on neurogenesis and BDNF production.”
In another part of the study, the investigators found that blocking neurogenesis in Alzheimer’s mice shortly after birth led to more pronounced cognitive deficits later in life. “We will next explore whether safely promoting neurogenesis in Alzheimer’s patients will help alleviate the symptoms of the disease and whether doing so in currently healthy individuals earlier in life can help prevent symptoms later on,” said Tanzi, the Joseph P. and Rose F. Kennedy Professor of Neurology at HMS. “We are very excited to now investigate ways of implementing our new findings to more effectively treat and prevent this terrible disease.”
Additional co-authors of the Science paper are Enjana Bylykbashi, Zena Chatila, Eunhee Kim, Alexander Rompala, Mary Oram, Caroline Asselin, Jenna Aronson, Can Zhang, Sean Miller, Andrea Lesinski, and Doo Yeon Kim, Genetics and Aging Research Unit, MGH Neurology; Benjamin Pulli and John W. Chen, MGH Radiology; Star W. Lee, Gregory Clemenson, and Fred H. Gage, Salk Institute for Biological Studies; Henriette van Praag, Florida Atlantic University; and Bruce M. Spiegelman, Dana-Farber Cancer Institute.
The study was supported with grants from the Cure Alzheimer’s Fund, the JPB Foundation, the Mathers Foundation, the Leona M. and Harry B. Helmsley Charitable Trust, and National Institutes of Health grants R01 MH060009, 1RF1 AG048080-01 and 2R01 AG014713.