Closer to detecting when and why blood clots form
Novel device can predict when a blood sample will obstruct the blood vessel network
More like this
Scientists at the Wyss Institute have created a better assay for testing blood’s clotting tendency, which could prove to be a lifesaver for patients with abnormal blood coagulation and platelet function.
As reported in today’s Nature Communications, this bioinspired advance by the Wyss Institute for Biologically Inspired Engineering at Harvard University takes a biophysical approach by subjecting blood to what it would experience inside a patient’s vascular network. It can be used with blood samples or potentially be integrated into patients’ blood-flow lines, offering clinicians the foresight they need to prevent life-threatening blood clotting or internal hemorrhaging.
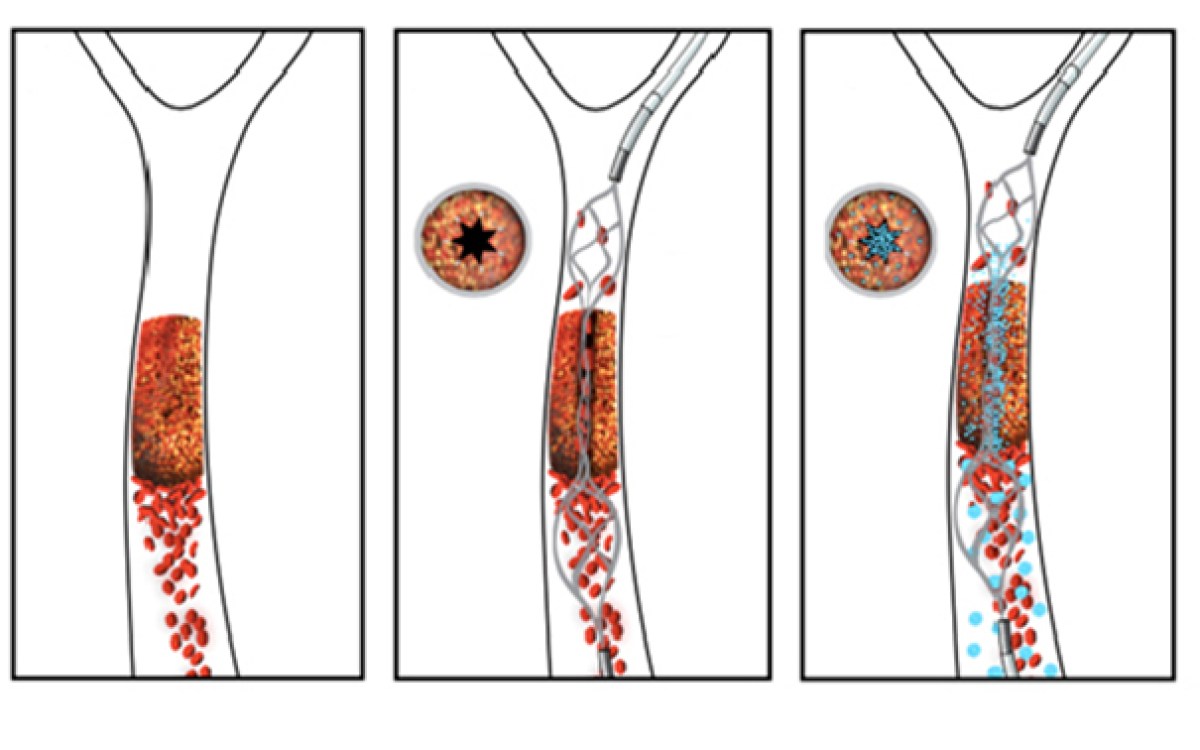
Led by Donald Ingber, founding director of the Wyss, the team has developed a novel microfluidic device in which blood flows through a lifelike network of small “vessels.” It is here that it’s subjected to true-to-life shear stresses and force gradients of the human vascular network. Using automated pressure sensors and a proprietary algorithm developed by the Wyss team, data acquired from the device is analyzed in real time, precisely predicting when a certain blood sample will obstruct the blood vessel network.
The device’s hollow channels mimic the pathology of the narrowing of small blood vessels, which occurs in patients as a side effect of medical conditions or treatments and can often cause a shift in the fluid mechanics of blood flow, possibly leading to life-threatening blood clots or internal bleeds.
Known clinically as hemostasis, the body’s ability to stop bleeding is critical for survival. For a patient with a blood-clotting disorder or medical condition, it is essential that care providers have the ability to quickly monitor the patient’s ability to maintain healthy hemostasis while preventing clotting.
“By combining our fabricated microfluidic device that mimics blood-flow dynamics of small arterioles with our novel data-analysis software, we can rapidly quantitate hemostasis in real time and predict if blood clots will develop in an individual or blood sample,” said Ingber, who is also the Judah Folkman Professor of Vascular Biology at Harvard Medical School, a member of the Pathology, Surgery and Vascular Biology Programs at Boston Children’s Hospital, and professor of bioengineering at the Harvard John A. Paulson School of Engineering and Applied Sciences.
The real-time monitoring ability of the device could also assess patients’ coagulation status almost continuously, in stark contrast to today’s standard of once- or twice-daily testing procedures, thereby reducing the likelihood of toxic side effects resulting from anticoagulation therapies. The team also demonstrated that the device could detect abnormal platelet function in patients with a rare bleeding disorder that is not easily identified using conventional assays.
“The physics of what’s happening inside our bodies is a major contributor to the reasons why blood clots form or why clotting fails during surgeries, traumas, or extracorporeal medical procedures,” said Abhishek Jain, a postdoctoral fellow at the Wyss Institute and the Division of Hemostasis and Thrombosis at Beth Israel Deaconess Medical Center and Harvard Medical School, the lead author on new study. “By mimicking the physics of blood clotting in our device more precisely, we hope this technology can one day be used to save lives.”
In a large animal experiment already conducted, the team perfused blood directly from a living vessel into a microfluidic device to measure clinical clotting parameters over time. From this research they recorded precise predictions for clotting times for blood samples, which were far more accurate and faster than currently used clinical assays.
According to the Wyss Institute, its hemostasis-monitoring device has been developed with translation and versatility in mind, using inexpensive in-line pressure sensors to measure clot formation. As a result, the device does not require additional instrumentation and can be integrated directly into the blood lines of extracorporeal devices.