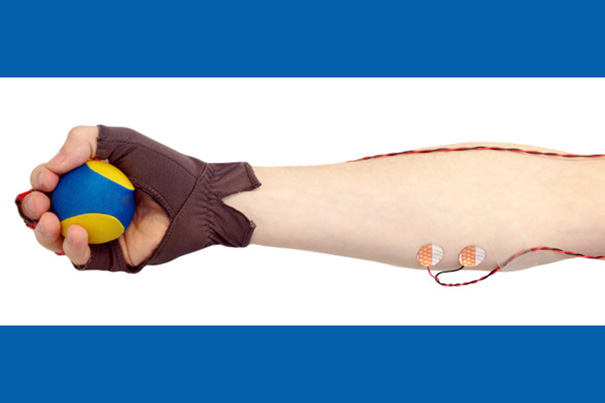
Sleep study participants are asked to squeeze a small ball in time with their breathing, and the force and timing of their motions are measured by the glove and by electrodes on a forearm muscle. By combining the information collected from the way squeezes decrease in frequency and strength as a participant falls asleep, investigators can now track the dynamic changes in brain and body during the sleep onset process.
Michael Prerau/MGH
Tracking the Sandman
MGH develops new system for measuring brain, body changes as people fall asleep
Investigators at the Harvard-affiliated Massachusetts General Hospital (MGH) have developed a system to accurately track the dynamic process of falling asleep, something that has not been possible with existing techniques.
In their report in the October issue of the open-access journal PLOS Computational Biology, the research team described how combining key physiologic measurements with a non-disruptive behavioral task gave them a better picture of the gradual process of falling asleep. In addition to being a powerful tool for future research, the system could provide new insight into diagnosing and understanding sleep disorders.
“While our personal experience tells us that falling asleep is a gradual process, current clinical methods only define a single point in time at which one has fallen asleep,” said lead author Michael Prerau of the MGH Department of Anesthesia, Critical Care, and Pain Management. “Our new research shows that it’s not simply when you fall asleep that’s important, it’s how you fall asleep that really matters. We now have the power to chart the entire trajectory of your neurological, physiological, and behavioral activity as you transition from wake to asleep, rather than simply reporting the time it takes.”
The new method continuously estimates the degree to which an individual is awake at each point during the sleep-onset process. “This is a real paradigm-shift in the way we study sleep onset,” said Patrick Purdon, MGH Department of Anesthesia, Critical Care, and Pain Management, the senior author of the study. “By quantifying the dynamic changes in brain activity and behavior during the transition from wakefulness to sleep, we now have a rigorous framework with which to study disorders of sleep onset, such as insomnia or narcolepsy.”
To link changes in brain activity to loss of consciousness during sleep onset, the investigators developed a new, minimally disruptive way to track behavior as subjects dropped off. Earlier methods either required participants to respond to auditory cues, which itself could disrupt sleep onset, or actigraphy, the method of measuring movement used in most clinical sleep devices and consumer wearables, which cannot distinguish between sleep and motionless wakefulness.
To get around these problems, the investigators developed an ingenious behavioral task that takes accurate measurements without disturbing sleep. Instead of responding to a sound, study participants hold a small rubber “stress ball” in one hand, which they are asked to squeeze with every intake of breath, and release with each exhalation. A special glove on that hand and electrodes on the forearm measure the timing and force of each squeeze. In this way, the participant’s own breathing acts as the stimulus, and the squeezes act as the behavioral response. Tracking how well the squeezes align with the subject’s breathing reflects a gradual process during which more and more squeezes are mistimed or absent. Measuring the force exerted by the forearm muscle reflects how the strength of the squeezing motion drops with sleep onset.
At the same time the ball-squeeze measurements are taken, EEG readings track three brain-wave patterns previously associated with falling asleep: decreasing power in the alpha frequency range, and increasing power in delta and theta frequencies. The combination of all these measures is used to calculate what the investigators call the “wake probability,” an estimate of the degree to which a participant remains awake as sleep sets in.
Testing their model in healthy volunteers over several nights not only provided more accurate results than traditional methods, it also revealed differences in the way sleep onset occurs in different individuals. Current clinical criteria define sleep as beginning when the power of an individual’s alpha-range brainwaves disappears. While seven of the nine study participants followed this pattern, two participants continued to correctly time their ball squeezes for several minutes after alpha levels had dropped. Only when the power in their brainwaves at the theta and delta frequencies had risen did both the behavioral and physiological measures indicate that they were asleep.
“These participants continued to respond to the task, even though current clinical measures would say they were still asleep, which was clearly not the case,” said Prerau. “These results suggest that it is the presence of delta and theta power, rather than the lack of alpha power, that is necessary for the cessation of behavior. We may need to carefully re-examine the way sleep onset is defined, since behavior is an essential component of the story that is not measured clinically.”
Prerau and Purdon believe their study will ultimately shed light on what happens in patients who have trouble falling asleep, leading to an improved ability to understand and diagnose sleep disorders, as well as to more precisely measure the effect of sleep medications. Their method could also be used to track drowsiness in situations in which alertness is vital.
Prerau is an instructor of anesthesia, and Purdon is an assistant professor of anaesthesia at Harvard Medical School. Additional co-authors are Katie Hartnack, Gabriel Obregon-Henao, and Aaron Sampson, MGH Department of Anesthesia; Margaret Merlino, Karen Gannon, and Matt Bianchi, MGH Department of Neurology; and Jeffrey M. Ellenbogen, Johns Hopkins University.





